In the pharmaceutical industry FTIR is a widely used technique for the analysis of solid and liquid medication. Applications include the analysis of polymorphic forms and solid-state characterization of active pharmaceutical ingredients (APIs). Additionally, FTIR plays an important role in the identification of impurities and contaminants in a quick and efficient manner. Why is that relevant?
Issue of impurities in medication
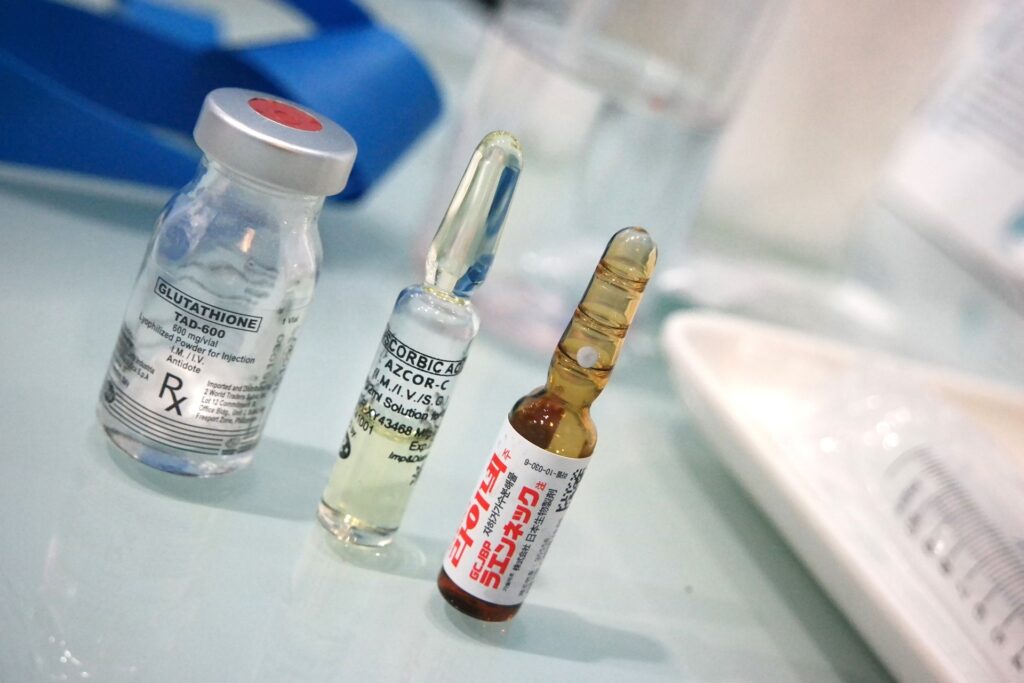
The purity of medication is crucial for ensuring its safety and efficacy. Particles present, for example in liquid pharmaceuticals, can cause physical and chemical changes in the drug formulation. This can lead to adverse effects such as irritation, inflammation, or allergic reactions.
In addition, particles can interfere with the drug’s intended mechanism of action. Particles can also affect the stability and shelf-life of the medication, leading to degradation or loss of potency over time. To prevent this, medications are closely monitored. Among other things, with FTIR.
Why FTIR?
When you want to detect and identify particulate matter, optical microscopy scores with high spatial resolving power. However, it fails to resolve the chemical composition. In contrast, FTIR microscopy not only provides high resolution but also comprehensive chemical information. This can be used to identify found particles. Furthermore, FTIR is a method recognized as a suitable tool for the characterization of particulate matter.
In the following, we will show how particles can be analyzed by µ-FTIR and Focal plane array detector (FPA), using liquid drugs as an example. Additionally, we will discuss which method proves to more efficient.

FTIR microscopy approach
Here an antibody solution contaminated with particulates was analyzed. In the first step, the particulate matter had to be separated from the liquid phase by filtering. Then an overview image of the filter was automatically acquired with the high-res visual camera of the LUMOS II FTIR microscope.
Based on their visual contrast, particles were detected using the Bruker Particle Finder routine. This revealed the presence of ten particles. A measurement point was automatically assigned to each found particle. The detected particulates were numbered, enlarged and superimposed onto the visual overview image (Fig. 1).
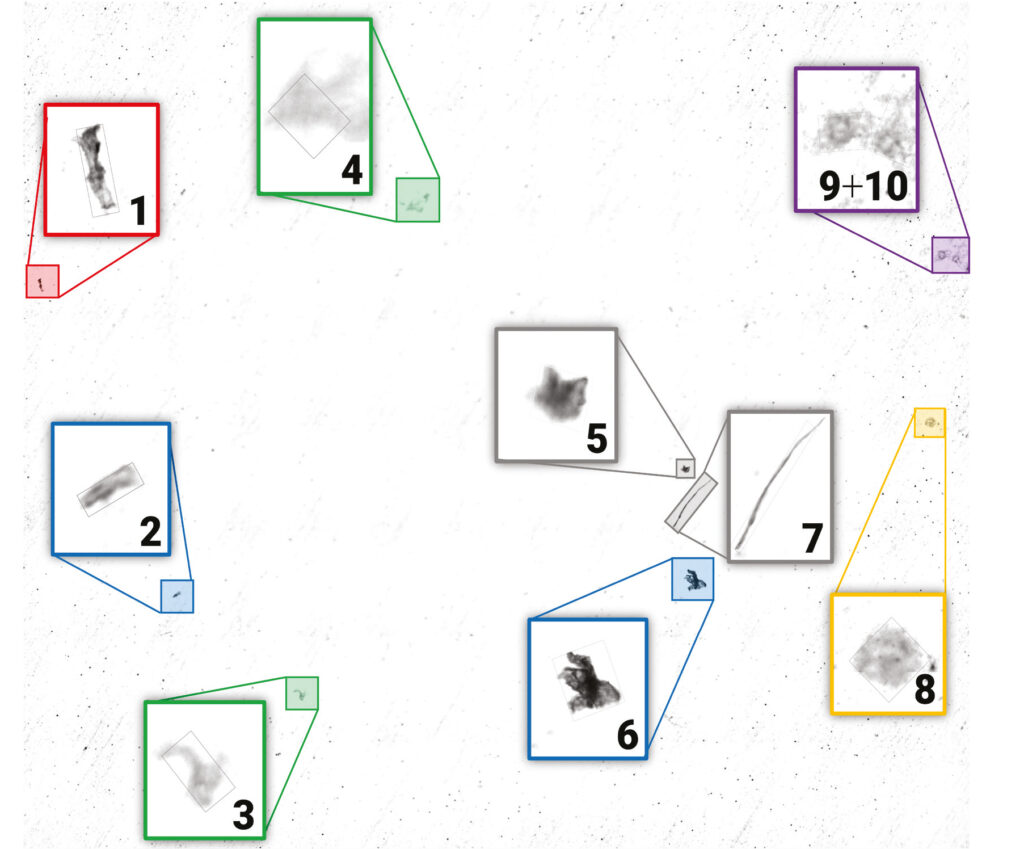
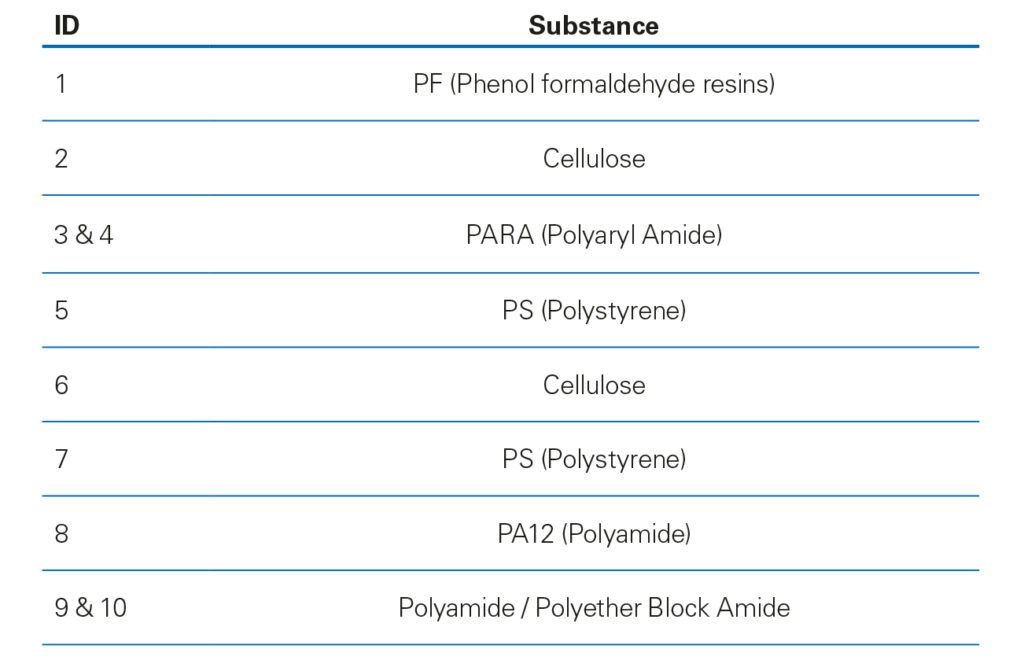
In a fully automated workflow, a μ-FTIR spectrum was collected at each particle ́s location by a transmission measurement. Every single particulate is identified successfully by the OPUS Cluster ID based on its FTIR spectrum (Table 1).
FPA imaging approach
The same antibody solution as above was used for this approach. Here, instead of using conventional FTIR μ-spectroscopy we utilized the FPA detector of the LUMOS II in transmission to create a chemical image. Since FPA detectors facilitate IR imaging at the highest speeds, the entire region of interest was measured in less than a minute and with a spatial resolution of 5 μm/pixel.
The dataset was processed completely autonomously using Bruker’s Adaptive Chemical Imaging AI algorithms and several clusters of particulates were identified (Fig. 2). The fiber visible to optical microscopy was identified as cellulose (pink), and two other visible particles were found to be polystyrene particulates (turquoise).
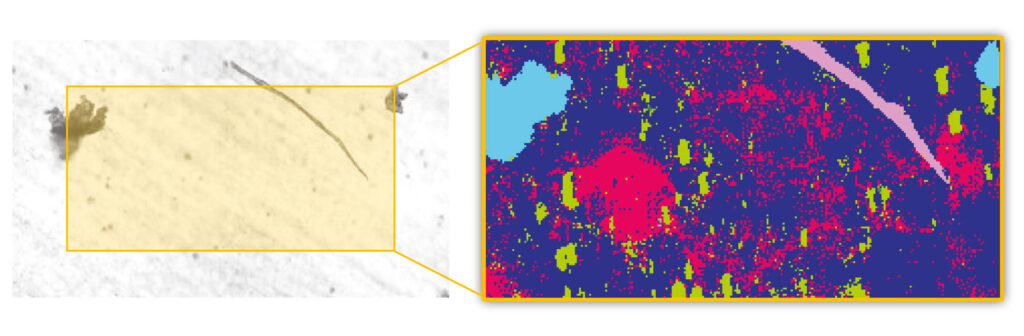
However, in comparison to the conventional FTIR μ-spectroscopy the imaging approach reveals much more. In particular, silicone oil (red) and protein clusters (green) that were initially invisible were successfully detected and identified, as shown in Figure 2. Both the detection and the identification of the particulates was solely based on the collected FTIR signature of the particles. This means that no particle remains undetected.
Conclusion
Infrared chemical imaging with the LUMOS II FPA detector is by far superior to the commercially available μ-FTIR methods for microparticle analysis. It delivers a perfect combination of high-speed and high-resolution chemical imaging. Here the chemical composition of the sample is deciphered independent of the contrast of the visible image. This is a major advantage of FTIR imaging compared to optical microscopy. For the investigation of particulates in injectable solution and microparticle analysis in general, we strongly recommend FPA chemical imaging as the superior method.
Patients rely on medication to manage their health conditions. Ensuring that the medication they receive is pure and of high quality is essential. This makes FTIR imaging an invaluable tool for quality control and assurance. We at Bruker are happy to be a part of this important task.
In case you want to know more about the use of FTIR in pharmaceutical industry have a look at this blog article about antibody stability:
COVID Cocktails and Antibody Stability